With your new medical device in mind, the process kicks into high gear in finding the right material suppliers and contract medical device manufacturers to work with. Everything from the components to mass production is a critical decision for a successful launch. Taking a product to market is a complex process that requires planning, collaboration, attention to detail, and above all else, communication.
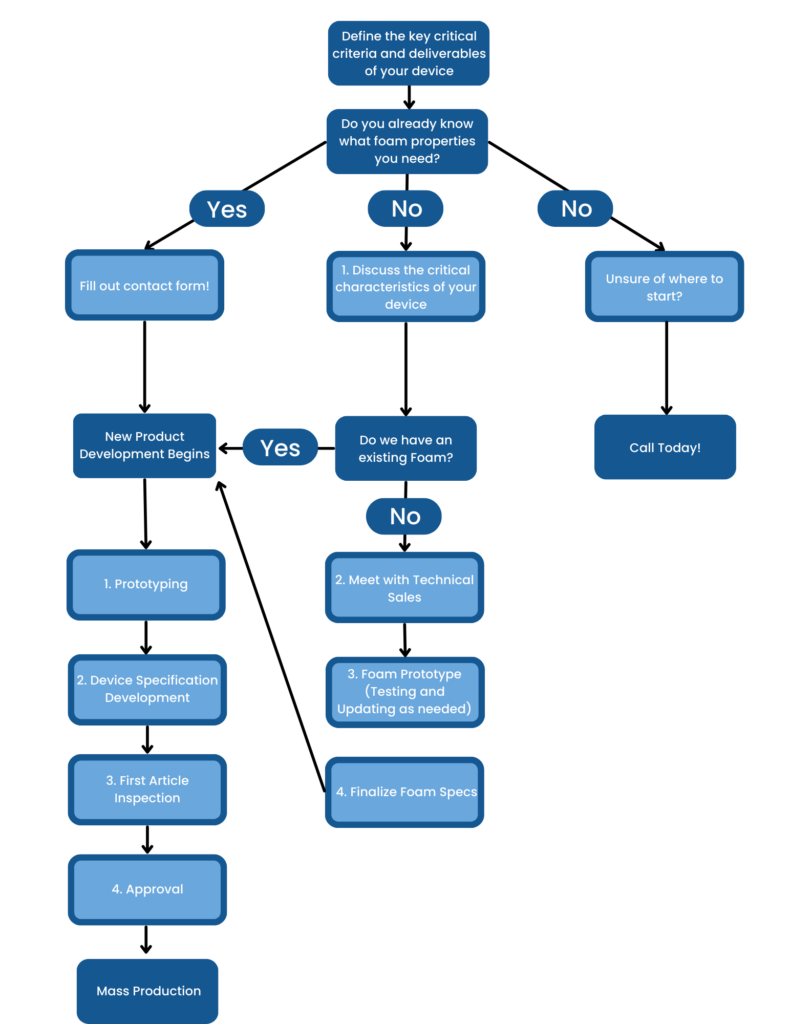
Foamtec, the leading vertically integrated medical device manufacturer, employs a comprehensive process development approach to ensure that your product meets your critical criteria and the highest, consistent quality. The Process Development Flow chart includes the following steps:
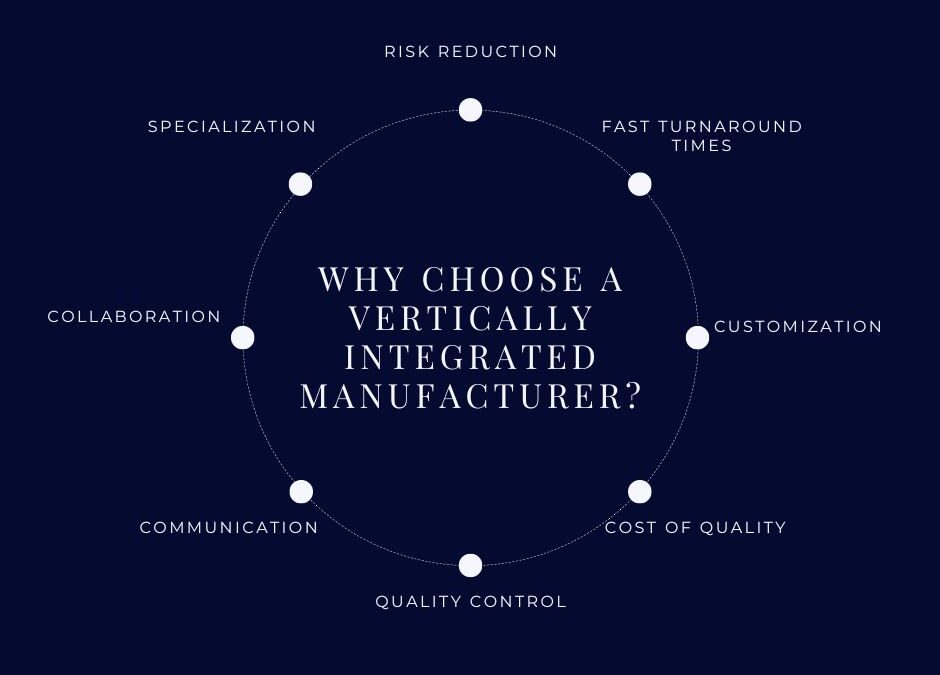
Why Choose a vertically integrated manufacturer?
- Risk reduction – Total traceability of all materials from base chemistry to final device is essential to provide minimal risk. Foamtec follows strict cGMP to provide assurance that we deliver the exact specifications that are agreed prior to any manufacturing.
- Fast turnaround times – Companies with in-house R&D, Engineering, Sales offer technical assistance you may not have in place yet. Mid-size companies may have the resources you need but also be able to give you the time and attention you are looking for. Can they produce and deliver components to meet tight deadlines?
- Customization – Differentiation in your market is why you are creating a new device. Application-specific raw materials give you an edge over competitors that make their devices from off-the-shelf material suppliers. Vertically integrated manufacturers like Foamtec offer diversity in materials and fabrication capability to provide you that differentiation.
- Cost of quality – Reliability, repeatability and the ability to create measurables ensure your device meets all of your and your customer’s critical needs. The cost to work with a supplier with validated processes is an intangible that is measured in zero internal and external customer complaints.
- Quality control – You expect reliable components that perform as expected. Understand the quality programs in place and define the ISO certification, compliance, traceability, and consistency you need. Quality Management systems ensure compliance from development to production to shipping. They also oversee documentation, testing, and validation.
- Communication – This is probably the most underestimated factor in success, and it’s a two-way street. How accessible is your supplier? When you see the prototype, are you concise and timely in your response? Do they respond to your changes and understand why they are critical?
- Collaboration – Different experiences and perspectives help to design devices that are easier to use, more efficient, or more affordable. A great understanding of end use by the supplier ensures an understanding of the key deliverables.
- Specialization – Components can be highly specialized. Knowing the properties of the component can help you find a partner who can meet your needs.
Innovation depends on creativity to generate new ideas and solutions. There is tremendous value in thinking through a problem or opportunity with a team that is on your side. A supplier with a wide range of experience and understanding of medical devices can help you select the right design, components, and packaging.
If you look at the most common reasons a medical device fails to get to market, you soon see why you need reliable partners. Intellectual property issues are a major concern for many developers and startups. Medical device companies that are familiar with the regulatory landscape and can help your project reach the market by avoiding communication breakdowns, supply chain disruptions, and manufacturing delays will pay for themselves many times over.
When a Custom-Manufactured Component is the Right Decision
You may need a custom medical component if you need added safety or patient functionality regarding speed, accuracy, or efficiency. Custom parts can increase ease of use, or reduction of risk, or improve compliance.
Vertically integrated component companies provide clients with unique products that give them quality and competitive advantages in the marketplace. The consistency, quality, and traceability derived from concept to completion of your medical device can make a difference. From design to distribution, know your partner’s strengths and weaknesses.
Contact us if you would like to learn more about the Foamtec Way and how we can help you design your medical device.